Research
Ligand-induced transmembrane signal transduction regulated by EGFR
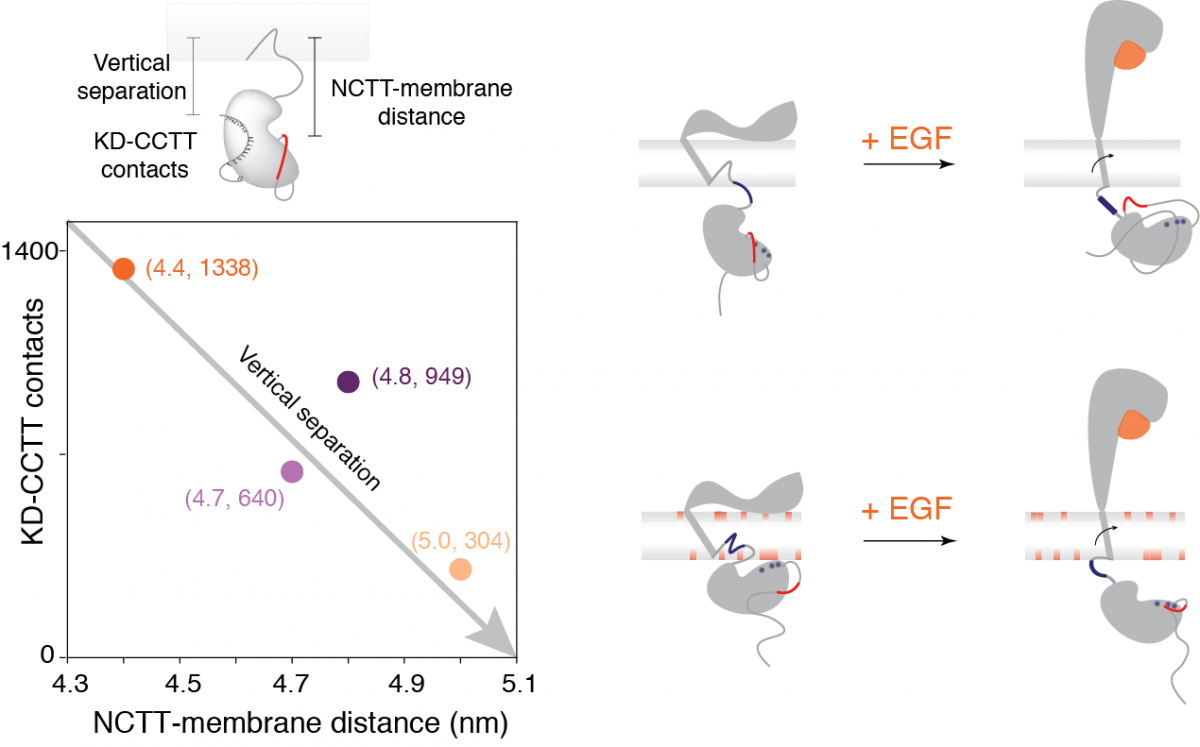 Receptor tyrosine kinases regulate signal transduction across cellular membranes. They control the communications between the intracellular environment and the extracellular world. As a prototypical receptor tyrosine kinase, epidermal growth factor receptor (EGFR), a single-pass transmembrane protein, is activated by binding of extracellular ligands. The aberrant expression of EGFR was implicated in many diseases such as cancer and diabetes. In collaboration with Prof. Gabriela Schlau-cohen, we aim to understand the mechanism behind the ligand-binding induced transmembrane signal transduction by EGFR.
Receptor tyrosine kinases regulate signal transduction across cellular membranes. They control the communications between the intracellular environment and the extracellular world. As a prototypical receptor tyrosine kinase, epidermal growth factor receptor (EGFR), a single-pass transmembrane protein, is activated by binding of extracellular ligands. The aberrant expression of EGFR was implicated in many diseases such as cancer and diabetes. In collaboration with Prof. Gabriela Schlau-cohen, we aim to understand the mechanism behind the ligand-binding induced transmembrane signal transduction by EGFR.
The concerted efforts between experiments and theory indicate a ligand-binding induced conformational change of monomeric EGFRs. This finding provides an alternative way for EGFR to transmit signals across the cellular membrane, in addition to the long-held picture of signaling by dimerization. My full-scale simulations reveal the molecular mechanism underlying this conformational transition, which is driven by electrostatic interactions. A positively charged juxtamembrane domain is the key element, whose interaction with the lipid bilayer and intracellular domain controls this structural transition.
Related publication:
Shwetha Srinivasan†, Raju Regmi†, Xingcheng Lin, Steven D. Quinn, Wei He, Kermit L. Carraway, III, Matthew A. Coleman, Bin Zhang*, Gabriela S. Schlau-Cohen*, "Intracellular compaction induced by extracellular ligand-binding in epidermal growth factor receptor", Nature Communications, 2022
Shwetha Srinivasan, Xingcheng Lin, Xuyan Chen, Raju Regmi, Wei He, Kermit L. carraway, III, Matthew A. Coleman, Bin Zhang*, Gabriela S. Schlau-Cohen*, "Lipid Dependence of the Intracellular Conformations of Monomeric EGFR", in preparation